Abrasion Resistant Porcelain Enamel
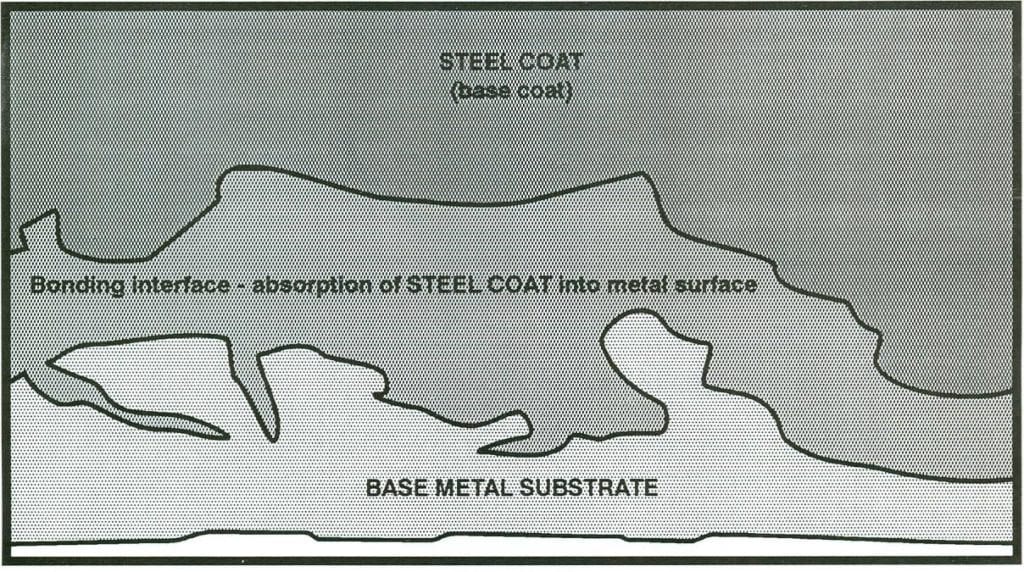
Steel Coat consists of special porcelains and inorganic materials applied in a minimum of two (2) coats, separately fixed to internal surfaces which are thoroughly grit blasted clean. Following application of the first (base) coat, the items shall be brought to a sufficiently high maturing temperature (above 1400F) to fuse the material to the base metal. Subsequent coatings will be processed in a similar manner, forming an integral molecular bond with the base coat and base metal. The finish lining shall be 8-12 mills thick and defects that expose the base metal shall be limited to 1 % of the total lined surface. Hardness shall be above 5 on the MOHS scale with a density from 2.5 to 3.0 grams per cubic centimeter. The lining shall be bonded sufficiently to the metal surface to withstand a .001 inch/inch (the yield point of carbon steel) without damage to the lining. The lining shall be capable of withstanding an instantaneous thermal shock of 350 F without crazing, blistering, or spalling. It shall be resistant to corrosion by solutions of between PH-3 and PH-10 at 1250F(Special formulations are available for specific higher or lower pH conditions).
Cutting of steel coat lined pipe shall be limited to only one piece per run of pipe for closure purposes, unless otherwise specified by the engineer. When manufacturer’s recommendations are followed carefully, spalling can be limited to a maximum of 1/8” back from the cut. Cuts should be made using a band saw with a lenox neo-type blade, ¼” wide x .025 thick x 18 teeth per inch, or finer, set at a speed of 100 ft. per minute. Insure the material is not forced against the blade, but set so that the cut is progressive in a natural way, chipping or spalling of steel coat is held to a minimum. Occasionally, a chip may go back .030 to .060”, but that is usually on an upward angle, leaving the substrate protected with a cover of ground coat. Pipe can also be cut with an abrasive high speed wheel. All supplied pipe and fittings will conform to AWWA and ANSI specifications.
call for additional product information.